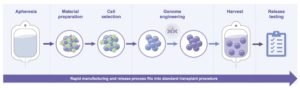
Speed—Rapid Manufacturing Cycle and Vein-to-Vein Time
In contrast to other patient-specific cell therapies, such as CAR-T therapies and gene-modified allogeneic cell therapies, our eHSCs manufacturing is a rapid and elegant process that is completed in approximately three days, enabling a seven-to-ten day vein-to-vein time. The primary reason we can produce eHSCs so quickly is the lack of a need for cell expansion. Our approach to creating eHSCs also does not involve the insertion of new genetic material, thereby avoiding complications related to the use of delivery modalities necessary for gene insertion, such as the viral vectors used in CAR-T therapies. The relatively simple and streamlined process of creating our eHSCs provides significant advantages in the required manufacturing infrastructure and we are developing in-house clinical current manufacturing capabilities to support our planned clinical trials. We believe the efficiency and low capital expenditure of our manufacturing process should translate into higher scalability, a lower cost of goods, and easy integration into routine transplant practice.
Investment in our own internal cell therapy manufacturing facility
In September 2022, we opened a new in-house clinical cell therapy manufacturing facility in Cambridge, Mass. co-located at our current headquarters. The new facility is designed to support development of our potentially transformative engineered hematopoietic stem cells (eHSCs) and CAR-T cell therapeutic candidates for patients with blood cancers.








